- Products
- Solutions
- Applications
- Accumulation
- Controls Solutions
- Custom Systems
- Depositing & Rejects
- Elevations & Line Egress
- Indexing & Positioning
- Inspection & Testing
- Merge, Diverting and Sorting
- Product Flow & Control
- Product Handling
- Warehouse Automation
- Industrial Conveyor Systems
- Lean Manufacturing and Warehousing
- Inline Labeling and Printing Conveyors
- Engineered Solutions
- Applications
- Industries
- Parts & Services
- Distributors
- Resources
- About Us
- D-Tools
- Careers
- Chat Live
- Blog
- Literature & Manuals
- Products
- Solutions
- Applications
- Accumulation
- Controls Solutions
- Custom Systems
- Depositing & Rejects
- Elevations & Line Egress
- Indexing & Positioning
- Inspection & Testing
- Merge, Diverting and Sorting
- Product Flow & Control
- Product Handling
- Rotating Conveyors
- Industrial Conveyor Systems
- Warehouse Automation
- Lean Manufacturing and Warehousing
- Inline Labeling and Printing Conveyors
- Engineered Solutions
- Applications
- Industries
- Parts & Services
- Distributors
- Resources
- About Us
Electronic Medical Device Manufacturing Solutions from Dorner Conveyors
Electronic Medical Device
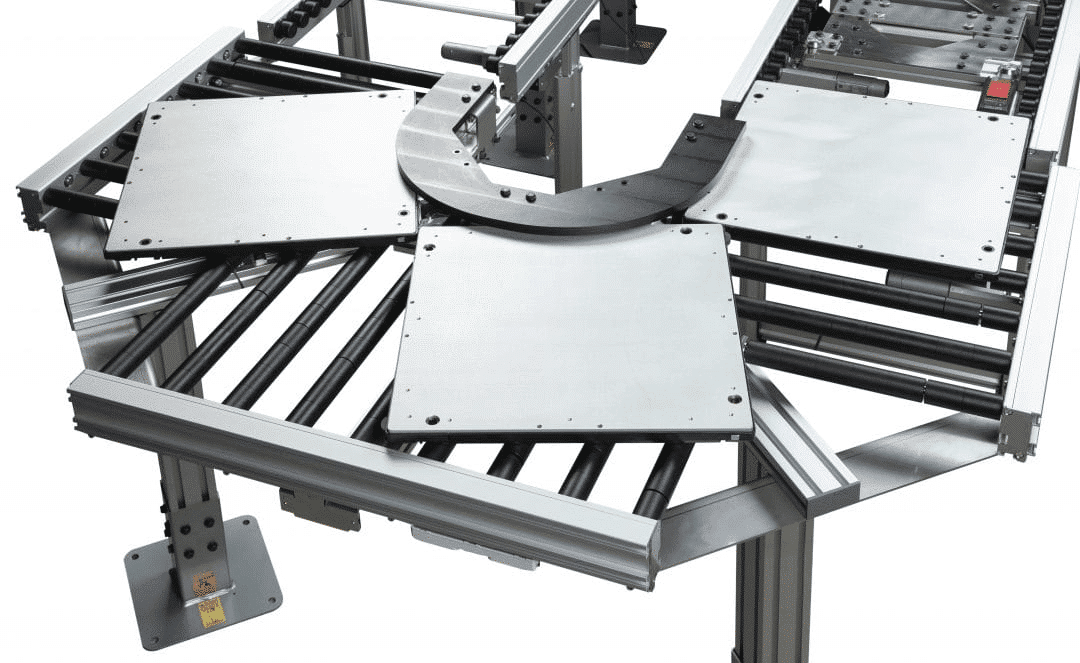
Meeting Industry Challenges Head-On
Miniaturization: As devices become smaller and more complex, manufacturing processes must adapt to handle components with microscopic precision.
Cleanroom Requirements: Many electronic medical devices, especially implantables, require pristine manufacturing environments to ensure patient safety.
Regulatory Compliance: Stringent FDA and IEC 60601 standards necessitate meticulous quality control and documentation throughout the production process.
Innovation at the Core
Benefits of Dorner Conveyors for Electronic Medical Device Manufacturing
Micron-level Precision for Miniaturized Components
- Smooth, consistent movement for even the tiniest components
- Precision positioning capabilities essential for micro-assembly processes
- Stable transport that minimizes vibration, crucial for sensitive electronics
Superior Cleanroom Compatibility
- Conveyor systems with ISO Class 4 cleanroom ratings, surpassing industry standards
- Materials and designs that minimize particle generation
- Easy-to-clean surfaces that support stringent sanitation protocols
ESD-Safe Environments for Component Protection
- ESD-safe materials and designs throughout our conveyor systems
- Rollers made of nylon and electrostatic dissipative materials
- Continuous grounding capabilities to prevent static buildup
Regulatory Compliance Support (FDA, IEC 60601)
- Conveyor systems designed with FDA and IEC 60601 standards in mind
- Documentation support for your compliance efforts
- Expertise in meeting cleanroom and particulate control requirements
Adaptability for Rapid Product Development Cycles
- Easy reconfiguration to accommodate new product lines or production changes
- Rapid integration of new technologies or processes
- Scalability to grow with your manufacturing needs
Data Integrity Maintenance for IoT-Enabled Devices
- Integration with electronic testing and calibration systems
- Reliable transport that doesn’t interfere with device programming or testing
- Compatibility with track-and-trace systems for complete product history
Longer Operational Life and Reduced Maintenance
- Mechanically driven rollers that outlast traditional timing belt systems
- Reduced downtime for maintenance and part replacements
- Lower total cost of ownership over the life of the conveyor system
Applications of Dorner Conveyors In Electronic Medical Device Manufacturing
Microelectronics Assembly for Implantables
- Ultra-smooth transport for delicate microcomponents
- Cleanroom-compatible designs to maintain sterility
- Precise indexing for accurate component placement
- ESD-safe materials to protect sensitive electronics
Automated PCB Handling and Testing
- Gentle handling to prevent board flexing or component damage
- Integration with automated testing equipment for seamless quality control
- Customizable layouts to optimize your specific PCB production flow
- Ability to handle boards of various sizes with quick changeovers
Cleanroom-Compatible Component Transport
- Reduced production to meet stringent cleanliness standards
- Sealed bearing designs to minimize potential contamination sources
- Compatibility with cleanroom protocols and cleaning agents
Integration with Electronic Calibration Systems
- Stable and consistent movement for accurate readings during calibration
- Easy integration with various calibration equipment
- Customizable conveyor layouts to create efficient calibration stations
- Ability to handle devices of different sizes and shapes
Packaging in ESD-Safe Environments
- Protection of devices from static discharge during packaging
- Smooth transitions between final testing, packaging, and shipping areas
- Integration with automated packaging systems for increased efficiency
- Maintenance of cleanliness standards through to the final packaging stage
Specialized Handling for Small, Delicate Components
- Capability to transport products weighing as little as a few grams
- Precision movement for components up to 35 lbs
- Ideal for small sensors, chips, and other miniature electronic parts
Facilitating Lean Manufacturing Processes
- Enabling efficient cell-based production layouts
- Facilitating quick changeovers for different product lines
- Supporting just-in-time production with flexible material handling
- Integrating seamlessly with robotic systems and other automation technologies
Dorner's Conveyor Solutions for Electronic Medical Device Manufacturing
Precision ERT150 Pallet Conveyors for Micro-Assembly
- Designed for micro-assembly and handling of delicate components
- Pallet-based system for secure transport of small, valuable parts
- Highly accurate positioning capabilities for integration with robotic systems
- Modular design for easy expansion and reconfiguration

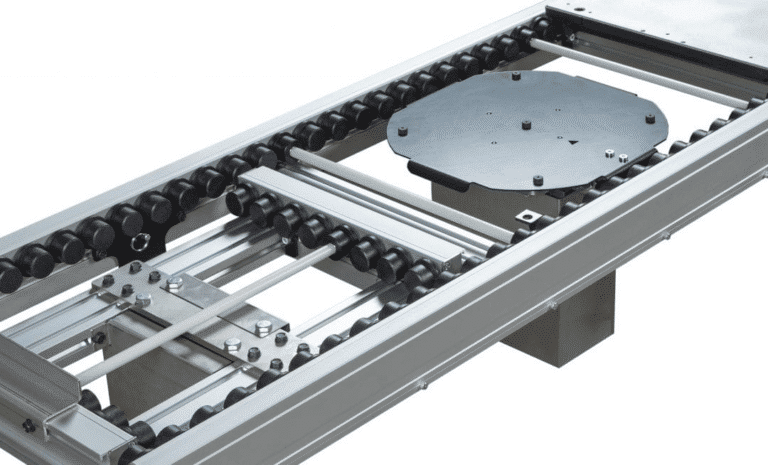
ERT250: Precision Engineered for Medical Electronics
- Superior cleanroom compatibility for the most sensitive production environments
- Mechanically driven rollers for precise, reliable movement without the limitations of timing belts
- Modular design allowing for easy customization and future expansion
- Ideal for cleanroom applications requiring the highest standards of cleanliness and precision
For more information on ERT conveyors, read our blog on their use in electronic medical device manufacturing.
FlexMove Conveyors
- >ESD-safe materials throughout to safeguard sensitive electronics
- Highly customizable layouts to fit your specific production needs
- Easy reconfiguration for changing product lines or production processes
- Seamless integration with existing equipment and workflows
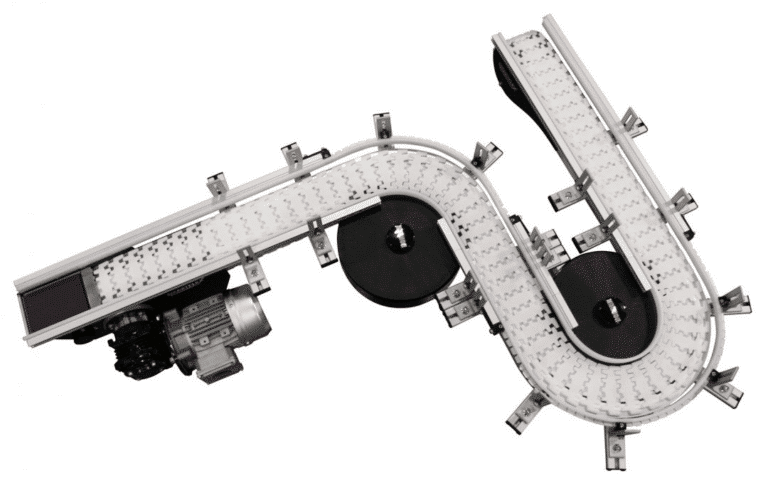

montrac® Shuttle Systems
- Verified for use in stringent cleanroom conditions up to ISO class 5
- Autonomous shuttle vehicles for precise, individualized product movement
- Easily adaptable tracks for changing production needs
- Ideal for complex assembly processes requiring multiple stations
1100 and 2200 Series: Versatile Solutions for Non-Sanitary Applications
- Ideal for passing components through machines or navigating small spaces
- Low-profile design for easy integration with existing equipment
- Versatile enough to handle a wide range of component sizes and weights
- Perfect for secondary packaging or transport between non-critical areas

Key Features Across Our Product Line
Modularity: All our systems are designed with modularity in mind, offering easily customizable layouts and a wide range of accessories for seamless integration with your existing processes.
Reconfigurability: As your production needs change, our conveyor systems can be quickly and easily reconfigured to meet new requirements, protecting your investment for years to come.
Precision: From the mechanically driven rollers of our ERT series to the autonomous shuttles of the montrac® system, every Dorner conveyor is engineered for the high-precision world of electronic medical devices.
Cleanliness: With superior cleanroom ratings and materials chosen for minimal particle generation, our conveyors maintain the strict cleanliness standards required in medical device manufacturing.
Top Electronic Medical Device Segments Benefiting from Dorner Conveyors
Smart Implantable Cardiac Devices
- Our ERT series provides the cleanroom-compatible environment crucial for these life-saving devices
- Precision handling ensures delicate components are transported without damage
- ESD protection safeguards sensitive electronics throughout the assembly process
Miniaturized Hearing Aids and Cochlear Implants
- This series excels in transporting and positioning micro-components weighing as little as a few grams
- Customizable layouts support the intricate assembly processes required for these devices
- Cleanroom-rated conveyors maintain the sterility needed for implantable components
Advanced Diagnostic Imaging Equipment Components
- Precision conveyance for delicate electronic parts used in ultrasound probes and other handheld diagnostic tools
- ESD-safe environments protect sensitive imaging sensors during assembly
- Modular designs adapt to the varied production needs of different imaging technologies
IoT-Enabled Patient Monitoring Systems
- Conveyor systems integrate seamlessly with electronic testing and calibration equipment
- Adaptable layouts support the assembly of various device types and sizes
- Our global support ensures consistent quality for manufacturers serving international markets
Robotic Surgical Systems Components
- High-accuracy positioning capabilities of our ERT series support integration with robotic assembly systems
- Modular designs adapt to the complex, multi-stage assembly processes of these sophisticated systems
Wearable Health Monitors
- Quick reconfiguration capabilities support fast product development cycles
- Gentle handling preserves the integrity of flexible electronics and sensors
- Scalable systems grow with your production as demand for wearables increases
Next-Generation Insulin Pumps and Continuous Glucose Monitors (CGMs)
- Precision conveyance for accurate positioning of micro-sensors and delicate components
- Cleanroom-rated systems ensure the sterility needed for these body-worn devices
- Modular designs support the integration of various assembly and testing stations
Neurostimulation Devices
- ESD protection throughout the conveyor system safeguards delicate neural interface components
- Precision movement supports the accurate assembly of miniaturized electrodes and circuitry
- Cleanroom compatibility maintains the high standards required for implantable devices
3D-Printed Prosthetics with Embedded Electronics
- Flexible conveyor layouts adapt to the unique production flow of 3D-printed components
- Precise handling supports the integration of electronic sensors and actuators into prosthetic structures
- Scalable solutions grow with your production as personalized prosthetics become more mainstream
Portable Diagnostic Devices (e.g., Handheld Ultrasound)
- expertly manages the transport of lightweight, delicate parts
- Modular systems support the varied assembly needs of different portable diagnostic tools
- Integration capabilities ensure smooth transitions between assembly, testing, and packaging stages
Partnering with Dorner for Electronic Medical Device Manufacturing Success
Cleanroom-Specific Customization
- Our team of experts works closely with you to design conveyor solutions that meet your specific cleanroom requirements
- We offer customized solutions that integrate seamlessly with your existing cleanroom setup
- Our conveyor systems are adaptable to various cleanroom classifications, facilitating compliance with your industry standards
Regulatory Compliance Expertise
- Our conveyor solutions are designed with cleanroom standards in mind
- We provide documentation to support your compliance efforts
- Our team stays up-to-date with evolving regulations to ensure our solutions meet the latest requirements
24/7 Support for Critical Production Lines
- Our global support network ensures assistance is always available, no matter where you are
- We offer rapid response times to minimize any potential disruptions to your production
- Our team provides remote diagnostics and support to quickly resolve issues
Continuous Innovation for Emerging Medical Technologies
- We invest heavily in R&D to stay at the forefront of conveyor technology
- Our modular designs allow for easy upgrades as your needs change
- We collaborate with industry leaders to anticipate future trends and develop solutions proactively
Proven Track Record
- We’ve been tested by demonstrations running continuously for over 21,000 hours (equivalent to 2.4 years of non-stop operation)
- Our global factory footprint allows us to support customers worldwide with consistent quality and service
- We leverage the combined power of Dorner and montratec® to offer comprehensive pallet system solutions
Industry-Specific Expertise
- Our team has extensive experience in the medical device manufacturing industry
- We understand the unique challenges and opportunities in this sector
- Our solutions are informed by real-world applications in leading medical device companies
Commitment to Your Growth
- Our customer-responsive approach means we’re always listening to your needs and adapting our solutions accordingly
- We offer scalable solutions that can grow with your business
- Our team provides ongoing consultation to help you optimize your production processes
Innovation Leadership
Frequently Asked Questions
How do Dorner Conveyors maintain cleanliness standards for implantable devices?
Can Dorner Conveyors integrate with electronic testing and calibration equipment?
How do Dorner's conveyors address ESD concerns in electronic medical device manufacturing?
Can Dorner conveyors be easily reconfigured as our production needs change?
What kind of support does Dorner provide for global electronic medical device manufacturers?
Contact Us

Dorner - Global Headquarters
Products
Industries
Quick Links
© 2024 Dorner Mfg. Corp. All Rights Reserved | Privacy Policy




